Isaiah 43:1-2 New King James Version (NKJV)
The Redeemer of Israel
43 But now, thus says the Lord, who created you, O Jacob,
And He who formed you, O Israel:
“Fear not, for I have redeemed you;
I have called you by your name;
You are Mine.
2 When you pass through the waters, I will be with you;
And through the rivers, they shall not overflow you.
When you walk through the fire, you shall not be burned,
Nor shall the flame scorch you.
And He who formed you, O Israel:
“Fear not, for I have redeemed you;
I have called you by your name;
You are Mine.
2 When you pass through the waters, I will be with you;
And through the rivers, they shall not overflow you.
When you walk through the fire, you shall not be burned,
Nor shall the flame scorch you.
Today is a new day and a better day. In my last post I was one miserable human being. The scripture above reminds me that through all I was going through and will continue to go through I can count on the Lord to help me weather the storm. In my deepest, darkest moments I called on him to ease my pain and he did. It kept coming back but each time I asked he would respond. As of today, I am absolutely getting better. I still have pain in my feet and hands although it is substantially less. There are a lot of things I cannot do using my hands due to their sensitivity to pressure. The pain in my feet have moved from the bottoms to the sides and I still cannot walk heel to toe. I have to walk flat footed or the friction from rubbing inside my shoes, even with socks on, causes pain. It's like having blisters on my hands and feet. My chest still burns and itches but it's more like an old sunburn. My nostrils are not as sensitive but still bleed when I blow my nose. My fingers are beginning to itch and I am hopeful that is a sign of healing.
We saw Dr. Arb, my oncologist on Friday and she told us that my body lacks an enzyme that metabolizes the (capecitabine) Zeloda. 1% of the population cannot take Zeloda and I am in that 1%. It's a genetic disorder. So, basically, the Zeloda was poisoning me and that's exactly how I felt. I likened it to Radiation poisoning. We asked about all the burning and itching and peeling of my skin and she said Zeloda goes after precancerous cells and any place I have had a sunburn the Xeloda will react with the skin. That explains why my chest, arms, top of the hands, face and lips were burning so bad. She likened it to the effects of Efudex used to treat skin cancer. (Note: The only genetic testing I had done was for the ovarian cancer gene and it was negative.)
EFUDEX: It is a cream that is applied to the skin where there is the presence of skin cancer cells. Wayne and my Step Dad have both used it. And, what happens is the skin will redden (severely in some cases), it makes the skin raw because it is eating through the layers and your skin looks like angry sores. It is very painful and burns really bad. Your face looks like a monster. You continue to apply the cream and gradually the cancer cells die, the skin heals and you're good to go.
So, the doctor said it will take 4 to six weeks for the effects of the medicine to go away. She has prescribed two new meds for me to take. My treatment plan is called AA Therapy. I will take Aromasin (exemestane) 25 mg. daily, once a day. It is available through my local pharmacy. And also take Afinitor, 10 mg. daily once a day. This medicine enhances the effectiveness of the Aromasin. I will also use a prescribed mouth rinse, Decadron (dexamethasone) 4 times daily to prevent mouth sores. The mouth sores are a common side effect and it is within the first 6 weeks they will show themselves. Also, a skin rash similar to acne may happen and the doctor will prescribe something for that should I experience it. I will take these meds continually without a break like the previous meds I was taking. There will be a high out of pocket cost for the Afinitor and we'll find out Monday what that will be. I will still get an Xgeva shot to protect my bones once every 3 months. I will follow up with the doctor again on Dec. 20 for labs and to assess my tolerability of the AA Therapy. I have a CT Scan scheduled for January, 2020.
Thanks for all the phone calls and prayers, it means a lot that so many have reached out to me. May God bless you all. If you didn't reach me by phone just know that I was overwhelmed and I was also sleeping a lot. I am looking forward to getting back to 'normal' whatever that may be.
I love you all,
Glenda
Here is info on the enzyme my body is lacking:
Dihydropyrimidine dehydrogenase deficiency
| Dihydropyrimidine dehydrogenase deficiency | |
|---|---|
| Other names | DPD deficiency |
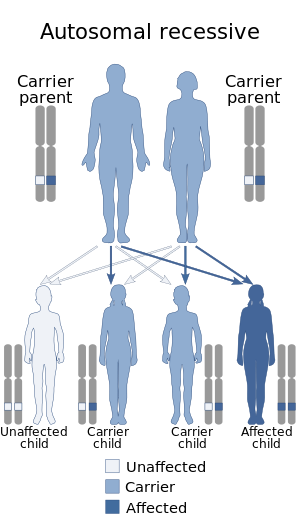 | |
| Dihydropyrimidine dehydrogenase deficiency has an autosomal recessive pattern of inheritance. | |
| Specialty | Medical genetics, endocrinology |
Dihydropyrimidine dehydrogenase deficiency is an autosomal recessive[1] metabolic disorder in which there is absent or significantly decreased activity of dihydropyrimidine dehydrogenase, an enzyme involved in the metabolism of uracil and thymine.
Individuals with this condition may develop life-threatening toxicity following exposure to 5-fluorouracil (5-FU), a chemotherapy drug that is used in the treatment of cancer.[2][3] Beside 5-FU, widely prescribed oral fluoropyrimidine capecitabine (Xeloda) could put DPD-deficient patients at risk of experiencing severe or lethal toxicities as well.[4][5]
Genetics[edit]
DPD deficiency is inherited in an autosomal recessive manner.[1] This means the defective gene responsible for the disorder is located on an autosome, and two copies of the defective gene (one inherited from each parent) are required in order to be born with the disorder. The parents of an individual with an autosomal recessive disorder both carry one copy of the defective gene, but usually do not experience any signs or symptoms of the disorder.
Diagnosis[edit]
Detecting DPD deficiency[edit]
A small number of genetic variants have been repeatedly associated with DPD deficiency, such as IVS14+1G>A mutation in intron 14 coupled with exon 14 deletion (a.k.a. DPYD*2A), 496A>G in exon 6; 2846A>T in exon 22 and T1679G (a.k.a. DPYD*13) in exon 13. However, testing patients for these allelic variants usually show high specificity (i.e., bearing the mutation means that severe toxicity will occur indeed)but very low sentivity (i.e., not bearing the mutation does not mean that there is no risk for severe toxicities). Alternatively, phenotyping DPD using ex-vivo enzymatic assay or surrogate testing (i.e., monitoring physiological dihydrouracil to uracil ratio in plasma) has been presented as a possible upfront strategy to detect DPD deficiency. 5-FU test dose (i.e., preliminary administration of a small dose of 5-FU with pharmacokinetics evaluation) has been proposed as another possible alternative strategy to secure the use of fluoropyrimidine drugs.
Epidemiology[edit]
Current research suggests that nearly 8% of the population has at least partial DPD deficiency. A diagnostics determination test for DPD deficiency is available and it is expected that with a potential 500,000 people in North America using 5-FU this form of testing will increase. The whole genetic events affecting the DPYD gene and possibly impacting on its function are far from being elucidated, and epigenetic regulations could probably play a major role in DPD deficiency. It seems that the actual incidence of DPD deficiency remains to be understood because it could depend on the very technique used to detect it. Screening for genetic polymorphisms affecting the DPYD gene usually identify less than 5% of patients bearing critical mutations, whereas functional studies suggest that up to 20% of patients could actually show various levels of DPD deficiency.
Women could be more at risk than men. It is more common among African-Americans than it is among Caucasians.[6]
No comments:
Post a Comment